Covaxin Who Approval Status Today
WHO panels four-day meeting begins today. 20 hours agoThe World Health Organization has sought clarification from Bharat Biotech on its Covid-19 vaccine Covaxin and will meet on Wednesday for a final risk-benefit analysis for its Emergency Use Listing.

Bharat Biotech S Covaxin Likely To Get Who S Emergency Use Approval Today
He also stressed that in a few days India will achieve administration of 1.

Covaxin who approval status today. 15 hours agoEarlier today Bharat Biotech said the Central Drugs Standard Control Organisation CDSCO has approved the extension of shelf life of its COVID-19 vaccine Covaxin. 2 days agoLast Updated. Union Health Minister Mansukh Mandaviya on Tuesday said that approval for Hyderabad-based Bharat Biotechs COVID-19 vaccine Covaxin will be given on the basis of.
Krishna Ella chairman and managing director Bharat Biotech said that the pharmaceutical company is also exploring. The World Health Organization WHO today granted the emergency use listing EUL status to Covaxin which has been developed by Bharat Biotech in India. The foreign secretary further stressed that its a technical process and not an administrative or a political process.
Technical Advisory Group seeks additional clarification on Covaxin for Emergency Use Listing. Approval for Covaxin had earlier been delayed on multiple occasions with Bharat Biotech asked for additional information by the approving authorities. WHO seeks additional clarification from Bharat Biotech decision likely on November 3 The technical advisory group will now meet on November 3 for a final assessment of Covaxin.
Heres a list of countries that have approved the Bharat Biotech vaccine Covaxin. While the WHO has so far approved Covid-19 vaccines of Pfizer-BioNTech AstraZeneca-SK BioSerum Institute of India Johnson Johnson-Janssen Moderna and Sinopharm for emergency use Covaxins approval. 15 hours agoThe Technical Advisory Group of WHO which met today has recommended the Emergency Use Listing status for Bharat Biotechs Covaxin - a decision that will ease international travel and the export of the indigenous vaccine.
With this the WHO has approved six vaccines for the prevention of COVID-19. WHO approves COVAXIN for EUL. File image news COVID-19 vaccine.
Covaxin decision date to be confirmed says WHO Premium A health worker preparing a dose from a vial of Covaxin a Covid-19 vaccine. 14 hours agoNew DelhiBhubaneswar. It will remove uncertainty around overseas travel by Indians inoculated with this vaccine.
The decision on Covaxins WHO EUL could be expected sooner than later with the global health bodys Strategic Advisory Group of. It is likely that the decision in this regard will be issued by the global health agency today. The World Health Organisation WHO on Wednesday said it had approved Indian drugmaker Bharat Biotechs COVID-19 vaccine for emergency use paving the way for the homegrown shot to be accepted as a valid vaccine in many poor countries.
The WHO clearance is a significant step in Covaxin being accepted by foreign governments. 13 hours agoWith the approval the demand for Covaxin is expected to rise. 14 hours agoThe WHO is in the process of evaluating Covaxins clinical trial data for use of EUL.
Following the emergency use approval for Covaxin. The World Health Organizations WHO technical advisory group will meet on October 26 to consider granting Emergency Use Listing EUL to Bharat Biotechs Covaxin the. The Technical Advisory Group TAG on Wednesday recommended Emergency Use Listing EUL status for Bharat Biotech COVID-19 vaccine Covaxin to the WHO.
Foreign Secretary Harsh Vardhan Shringla has said that Bharat Biotechs Covaxin Indias first indigenous Covid-19 vaccine will be approved by the World Health Organisation WHO soon. Heres a list of countries that have approved the Bharat Biotech vaccine As the vaccine is yet to receive a clearance from the World Health Organisation Indians who have taken Covaxin miss out on travelling to countries like the UK USA among others. The World Health Organisations panel had last week sought additional clarifications from the Hyderabad-based pharma firm.
12 hours agoSoumya Swaminathan. Covaxin has demonstrated 778 per cent effectiveness against symptomatic COVID-19 and 652 per cent protection against the new Delta variant. Bharat Biotechs Covaxin and AstraZeneca and Oxford Universitys Covishield are the two widely used vaccines in India.
The WHO Technical Advisory Group for Emergency Use Listing EUL is an independent advisory group that provides recommendations to WHO on whether a Covid-19 vaccine can be listed. 15 hours agoOman last week and Australia on Monday said they will recognise Covaxin as a valid vaccine for travellers. 15 hours agoCovaxin WHO approval.
Bharti Pawar thanked PM Modi for the Covid vaccination drive and said that today its a joyous day for Indians. 15 hours agoThe Technical Advisory Group of the WHO recommended Emergency Use Listing status for Bharat Biotechs Covaxin after the company submitted a few more documents. 12 hours agoCovaxins approval came after the Technical Advisory Group TAG an independent advisory committee of WHO recommended EUL status for the vaccine made by Bharat Biotech.
23 hours agoBharat Biotechs Covaxin May Get WHOs Emergency Use Approval Today. The Technical Advisory Group TAG an independent advisory panel of WHO will most. 1st November 2021 1212 IST Australia Recognises Covaxin Day After PM Modi Pushes For WHO Approval Of Indian Vaccines In a massive victory for India Australia on Monday has recognised Covaxin as one of the recognised vaccines of travellers wishing to enter the state.
On October 18 the WHO had said that it was expecting one additional piece of information from Bharat Biotech on Covaxin. Here is A List Of Countries Where It is Approved The Technical Advisory Group TAG an independent advisory group that provides recommendations to WHO on whether a Covid19 vaccine can be listed for emergency use met on October 26 2021 and had asked for additional clarifications from the manufacturers to. The WHO has given approval to Pfizer-BioNTech AstraZeneca-SK BioSerum Institute of India Johnson 7 Johnson Janssen.
23 hours agoThe World Health Organisation WHO has already delayed the decision regarding the approval for Bharat Biotechs Covaxin for emergency usage by several weeks.
Bharat Biotech S Covaxin Gets Who Approval For Emergency Use Listing Business Standard News

Covaxin Approval Soon Who Chief Scientist Says Phase 3 Trial Data Looks Good Breaking News Youtube
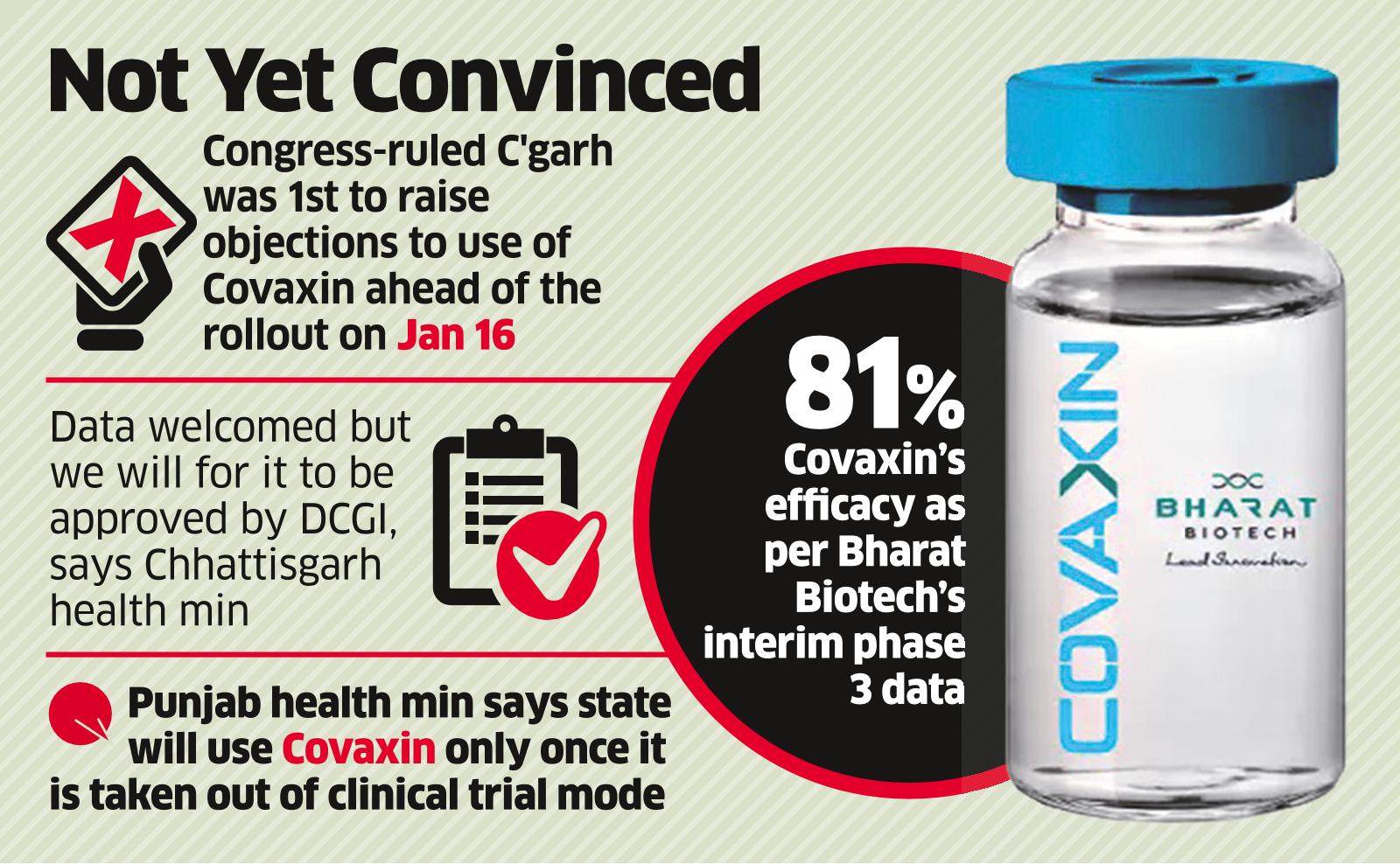
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times

Covaxin Bharat Biotech S India Made Covid Vaccine Cleared By Who Sources

Covid 19 Vaccine Covaxin Approval Who Seeks More Data From Bharat Biotech For Emergency Use Licensing The Financial Express

Covaxin Who Approval For Travel Status News Update More Updated November 2021 Wego Travel Blog

Who Grants Approval To Covaxin For Emergency Use Listing
Covaxin Here S A List Of Countries That Have Approved The Bharat Biotech Vaccine India News The Indian Express

Covaxin S Us Approval Delayed As Fda Asks For More Data India News Times Of India

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News
Coronavirus India Approves Covid 19 Vaccines Covishield And Covaxin For Emergency Use The Hindu
Covaxin S Lack Of Usfda Approval A Roadblock In Getting Who Green Flag The Hindu Businessline

Covaxin Approval Today Crucial W H O Meet Today To Decide On Nod For Bharat Biotech S Covaxin Youtube

Who To Consider Granting Eul For Bharat Biotech S Covaxin On Tuesday